Bio-geo-chemical cycles
Bio-geo-chemical cycles
A constant interaction, between the
biotic and abiotic components of the
biosphere, makes it a dynamic, but stable
system. These interactions consist of
transfer of matter and energy between the
different components of the biosphere.
Bio-geo-chemical pathways determine the
path of transfer of matter on earth. Let us
look at some of the major biogeochemical
cycles.
Bio-geo-chemical cycles as we may
see from the name itself includes both
biological, geological and chemical or
physicochemical pathways. This means the
reservoir or pool of nutrients on earth may
contain some chemicals of biological
origin while others may be purely inorganic
in nature also may be geochemical
(obtained from rocks and soil) in origin.
Water though not considered as a biogeo-chemical cycle by most ecologists
actually is the precursor of the major
elements Hydrogen and Oxygen as some
living organisms use them for making the
basic food molecules for several
organisms in nature.
Water is also a universal solvent and
essential for various reactions to take place
within a living cell. Thus we shall also take
up water cycle briefly in this chapter.
Though the nutrient pool involves several
elements of nature but, we shall study just
the cycling of some major elements like
Oxygen, Nitrogen and Carbon.
The water cycle
All the water that is on the earth has
always been here? Earth never gets water
added to it nor does water disappear from
the earth. Water is constantly recycled in a
process known as the Hydrological or
Water cycle.
Fresh water is more scarce than you
might think.Nearly 97% of all the water on
the earth is in the oceans, and so only about
3% is fresh water. About 2% of this fresh
water is permanently frozen in Glaciers and
at the Polar Ice caps.
Thus only about 1% is available fresh
water. Again about 1/4 of this 1% is present
as groundwater. Only about 0.009% of
water on earth is in the rivers and lakes.
Rest is present in the bodies of living
organisms, as soil moisture, as humidity of
atmosphere etc. Water is the most essential,
abundant substance in living things.
The human body for example, is
composed of about 70% water (remember
all living organisms together constitute
only 0.005% of water on earth). Water
participates in many biochemical
mechanisms, including photosynthesis,
digestion and cellular respiration. It is also
the habitat for many species of plants,
animals and microorganisms, and it
participates in the cycling of the materials
used by living things. So, it is important
that we protect our water
resources.
The Nitrogen cycle
Nitrogen is both the most abundant
element in the atmosphere and, a building
block of proteins and nucleic acids. The
Nitrogen cycle is a complex
biogeochemical cycle in which Nitrogen
is converted from its inert atmospheric
molecular form (N2
) into a form that is
useful in biological processes.
The element Nitrogen is constantly
moving in a giant circle from the air,
through the soil, into the bodies of plants
and animals, and eventually back to the air
by the process of Nitrogen cycle. All living
things need nitrogen mainly for growth,
repair and development (Nitrogen being
essential for protein formation). Even
though the Earth’s atmosphere is made up
of 78% nitrogen, plants and animals cannot
use it in this form.
The atmospheric nitrogen is thus
converted into certain compounds which
plants may take up from the soil by some
biochemical process (caused by certain
bacteria like Rhizobium, Nitrosomonas etc)
and physicochemical processes (caused by
lightning). Animals get the required amount
of nitrogen from plants either directly
(herbivores) or indirectly (carnivores).
The nitrogen cycle contains several
stages:
1. Nitrogen fixation:
Atmospheric nitrogen occurs primarily
in inert form (N2) or non reactive form that
few organisms can use; therefore it must
be converted into a compound - or fixed -
form in a process called nitrogen fixation.
Most atmospheric nitrogen is ‘fixed’
through biological processes. A number of
bacteria and blue green algae are known to
be able to fix atmospheric nitrogen into
compounds in their own body. These may
be symbiotic (Rhizobium) or freeliving
(Nitrosomonas) respectively. These
organisms convert atmospheric nitrogen
into the organic nitrogen for their own
cells. As they die rapidly( they grow rapidly
as well), this nitrogen, now present in the
soil as compounds become available to
plants. In leguminous plants like pea, beans
etc there is a symbiotic relationship of the
nitrogen fixing bacteria with the plant.
2. Nitrification:
Nitrates can also be converted to
Ammonia by the de Nitrifying Bacteria in
the soil (especially in waterlogged soils).
The nitrifying bacteria may then use this
ammonia to synthesize compounds for
their own cell and eventually convert to
Proteins, Nucleic acids, Nitrites and
Nitrates. Nitrites are produced mainly by
Nitrosomonas, while nitrates by
Nitrobacters that are also capable of
utilizing nitrites and converting them to
nitrates. Death of these microorganisms
add the nitrogenous compounds to the soil.
Plants take up nitrate as well as ammonium
ions from the soil to convert them to
proteins and nucleic acids.
3. Assimilation:
Nitrogen compounds mainly as nitrates
or ammonium ions(NH4+) are taken up
from soil by plants which are then used in
the formation of plant proteins and as
animals eat these plants, animal proteins
are synthesised.
4.Ammonification:
Production of Ammonia (NH3
) from
Nitrates and other Nitrogenous compounds
in called Ammonification.
Describe a path of ammonification
discussed in the above section.
Ammonification also occurs when
plants and animals die, or when animals
emit wastes, the nitrogen in the organic
matter reenters the soil and water
bodies where it is broken down by
other microorganisms, known as
decomposers. This decomposition
produces ammonia which is then
available for other biological
processes.
5.Denitrification:
Nitrogen makes its way back into
the atmosphere through a process
called denitrification, in which
solid nitrate (NO3) is converted back to
gaseous nitrogen (N2). Denitrification
occurs primarily in wet soils where water
makes it difficult for microorganisms to
get oxygen. Under these conditions, certain
organisms - known as denitrifiying bacteria
- will process nitrate to gain oxygen,
leaving free nitrogen gas as a byproduct.
Thus, the nitrogen content of the earth
and its atmosphere remains in a perfect
balance.
Human intervention and nitrogen
cycle
Unfortunately, humans are interfering
with the natural balance when they overuse
artificially produced nitrates as agricultural
fertilizers that are often washed into water
bodies by rain as well as by releasing
exponential amounts of untreated domestic
sewage into water bodies. Before these
nitrates can be converted into atmospheric
nitrogen, they are often carried off by rain
or irrigation to streams and rivers and even
seep down to groundwater.
In some parts of the world, water for
humans and animals contains such high
concentrations of nitrates that it is unsafe
for consumption. These excessive amount
of nitrates and other nitrogenous
compounds, when they reach rivers and
lakes, cause too much algal growth. This
over-abundance of algae uses up too much
of the oxygen in the water. When oxygen
level falls, other forms of life in the water
bodies die off.
The Carbon cycle
Carbon is found in various forms on the
Earth. It occurs in the elemental form as
say Soot, Diamond and Graphite. In the
combined state, it is found as gases, Carbon
dioxide and Carbon monoxide in the
atmosphere, as carbonate and hydrogen
carbonate salts in various minerals, while
all life-forms are composed of carbon
containing molecules like Proteins,
Carbohydrates, Fats, Nucleic acids and
Vitamins. The endoskeletons and
exoskeletons of various animals are also
formed from carbonate salts.
Carbon dioxide is also responsible for
maintaining the Earth as a Green house with
temperature conditions suitable for life.
Thus, Carbon exists in the biosphere as the
central element of life. Carbon Dioxide or
CO2
, now makes up about 0.04% by volume
of air.
Have you ever thought how this level
of Carbon is being maintained in the
nature?
Carbon is incorporated into life
through various processes. The main
reservoirs of carbon are sedimentary rocks,
fossilized organic carbon including the
fossil fuels, the oceans, and the biosphere.
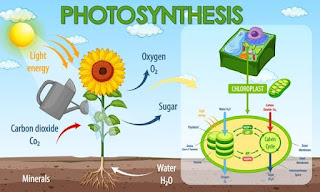
Photosynthesis:
The first step in the biological
carbon cycle is the conversion of
inorganic atmospheric carbon into a
biological form. This ‘fixing’ of
carbon in biological form takes place
within plants and other organisms -
known as producers - in a process
called photosynthesis, by which
energy from sunlight is converted into
chemical form.
In photosynthesis, light energy
helps to combine carbon dioxide and
water to create the simplest of sugars,
the carbohydrate molecules known as
Glucose (C6H12O6). In oceans,
photosynthesis is carried out by
microscopic aquatic plants called
phytoplankton. The carbohydrates
then become the source of chemical
energy to cells in all plants and
animals. In plants, some carbon
remains as simple glucose for short-term
energy use, while some are converted
to large complex molecules such as starch
for long term energy storage.
The Green house effect:
A greenhouse is a small house made of
glass that is used to grow plants. It traps
the sun’s rays and keeps the heat from
escaping. It is warm inside. In the same way
that the glass traps heat in a greenhouse,
some gases present in the atmosphere such
as Carbon di Oxide, Carbon monoxide,
Methane and Water vapour trap heat from
radiating back to the space. The natural
greenhouse gases act like a big blanket
around the earth, keeping it warm and
making life possible without which
temperatures would have fallen to sub zero
values. This phenomenon of naturally
warming up is called ‘‘Greenhouse effect”.
But the extent of this natural warming
up process have been grossly affected now.
Due to various human activities like burning
of fossil fuels, deforestation and
industrialization, an excessive amount of
carbon dioxide and other green house gases
has been emitted to the environment. As a
result more heat gets trapped. This causes
the temperature of the earth to rise, which
results in Global Warming.
Oxygen cycle:
Oxygen is an abundant element,
next to Nitrogen, on our Earth. It is
found in the elemental form in the
Atmosphere to the extent of nearly 21%.
It also occurs extensively in the combined
form in the Earth’s Crust as well as in the
air in the form of carbon dioxide. In the
crust, it is found as the oxides of most
metals. It is also present as carbonate,
sulphate, nitrate and other compounds. It
is also an essential component of most
biological molecules like carbohydrates,
proteins, nucleic acids and fats (or lipids).
The cycle and storage:
Oxygen from the atmosphere is used
up mainly by the processes, combustion,
respiration and in the formation of oxides
of elements like Nitrogen, Iron etc. Oxygen
is returned to the atmosphere in only one
major process, that is, Photosynthesis.
Ozone layer:
The Earth’s atmosphere is divided into
several layers. The lowest region, the
Troposphere, extends from the Earth’s
surface up to about 10 kilometers (km) in
altitude. Virtually all human activities occur
in the Troposphere. Mount, Everest, the
tallest mountain on the planet, is only about
9 km high. The next layer, the Stratosphere,
continues from 10 km to about 50 km.
Most commercial airline traffic occurs in
the lower part of the Stratosphere. most
atmospheric ozone is concentrated in a
layer in the stratosphere, about 15-30
kilometers above the Earth’s surface.
Ozone is a molecule containing three
Oxygen atoms. It is blue in colour and has
a strong odour.
Normal oxygen, which we breathe, has
two oxygen atoms and is colourless and
odourless. Ozone is much less common
than normal oxygen. Out of each 10 million
air molecules, about 2 million are normal
Oxygen, but only 3 out of 10 millions are
Ozone.






No comments:
Post a Comment